America
After Cough Syrup, Now Indian-Made Eye Drops Cause Concern; CDC Flags EzriCare
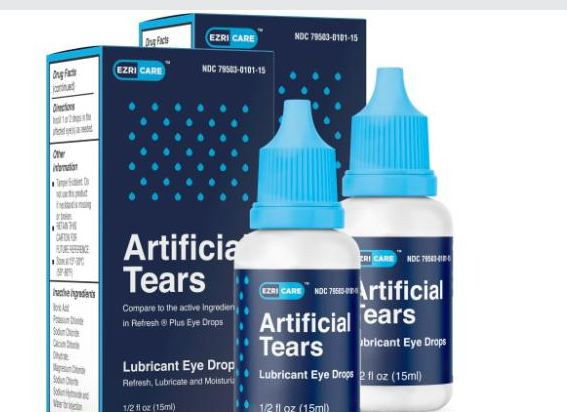
ATLANTA, GA: The US Center for Disease Control and Prevention (CDC) has flagged concern over the use of an Indian eye drop that is causing death and blindness.
The CDC has, in recent months, traced three deaths, eight cases of blindness and dozens of infections caused by a highly drug-resistant bacteria linked to EzriCare artificial tears, made by Global Pharma Healthcare, The New York Times reported.
Drug-resistant bacteria Pseudomonas aeruginosa has been a cause of concern among people with weak immune systems, residents of nursing homes and patients using catheters and breathing tubes.
In February, Global Pharma Healthcare recalled and stopped the production of both EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears. And the US Food and Drug Administration (FDA) had instructed doctors and consumers not to purchase the product from the market and warned those who have already purchased it not to use it.
The warning issued by the FDA read, “using contaminated artificial tears increases the risk of eye infections that could result in blindness and deathâ€.
However, the CDC said it was concerned that the bacteria could gain a foothold in the US, as the bacteria showed signs of spreading among asymptomatic patients, in a Connecticut care center, who had the bacteria colonized in their bodies. Such spread tends to occur when patients touch common items or when healthcare workers transmit the germs.
According to experts, pseudomonas is very hard to get rid of. Pseudomonas is especially difficult to eradicate, both from health care facilities, where it clings tenaciously to sink drains, water faucets and other moist environments, and from patients who develop bloodstream infectionsâ€, David van Duin, an infectious disease specialist at the University of North Carolina School of Medicine, was quoted as saying.
After the FDA had issued the warning in February, a group of drug inspectors from the Central and Tamil Nadu governments conducted an inspection at the company premises which is 40 km south of Chennai.
While refusing to comment on the FDA’s remarks on the eyedrops, the Tamil Nadu drug regulator now said it found “no contamination†in samples of eye drops manufactured by Global Pharma, media reports said.
This is the third such incident where an Indian pharmaceutical product has come under scrutiny.
Last year, dozens of deaths were reported among children in Gambia and Uzbekistan due to Indian cough syrups.
Cough and cold syrups manufactured by New Delhi-based Maiden Pharmaceuticals were linked to the deaths of at least 70 children in Gambia, while Delhi-based Marion Biotech’s cough syrups were linked to the deaths of 18 children in Uzbekistan.

5 hours ago
India criticises Pakistan heading UNSC committee on sanctions against Taliban

5 hours ago
Taliban says US drones entering from neighbouring region still patrolling Afghanistan skies

8 hours ago
Indian Navy chief discusses deepening maritime ties, Indo-Pacific security with top US officials

10 hours ago
Trump’s support for H-1B reignites debate over skilled-worker visas

10 hours ago
Bill to ban H-1B visas ‘efficient way to hurt Americans,’ says US Immigration expert

10 hours ago
$100,000 H-1B fee ‘significant step to stop abuse’: White House

10 hours ago
Trump drops tariffs on food imports; India’s mango, tea exports may benefit

12 hours ago
Guneet Monga Kapoor recollects when chef Vikas Khanna flew to New York to support Oscar campaign

12 hours ago
Selena Gomez talk about the dreamy marriage with Benny Blanco

12 hours ago
Parineeti Chopra gushes over Sania Mirza: ‘Why are you the world’s best?’

12 hours ago
Shreya Ghoshal announces her 'The Unstoppable Tour', calls it a celebration of her journey so far

12 hours ago
Mahesh Babu says 'Thinking of you a little more today' as he remembers late father Krishna

12 hours ago
Sanjay Dutt’s sister Priya shares a heartfelt memory of mother Nargis Dutt’s ‘centre of world’






















